Competent cells for transformation
- Home
- Competent cells for transformation
- Low Endoxin Cells
- ClearColi BL21(DE3) Electrocompetent Cells
Recommended
ClearColi BL21(DE3) Electrocompetent Cells
Product Details
Show HideEach ClearColi® BL21(DE3) Electrocompetent Cell Kit contains: ClearColi BL21(DE3) Electrocompetent Cells in DUO packaging (2 transformations per tube), Expression Recovery Medium (lactose minus), pUC19 Positive Control Plasmid, and complete protocols.
Expression Recovery Medium (lactose minus) is also available separately.
ClearColi BL21(DE3) Electrocompetent Cells
Key features
Show Hide- Eliminate endotoxin at the source
- Genetically modified LPS does not trigger endotoxic response in human cells
- Ideal for mammalian cell immunogenicity testing, toxicity assays, and therapeutic protein drug discovery
- Useful for membrane and lipid binding protein production
- Protein expression similar to BL21(DE3) cells without requiring downstream endotoxin removal
- Reduce false positives in cytokine assays, improve confidence in your results
Cannot add to favourites
An option must be selected in order to add to favourites
Similar Products
Phage Display Library Construction
ER2738 Electrocompetent Cells
Large or Difficult Cloning
TransforMax EPI300-T1R Electrocompetent Cells
Phage Display Library Construction
TG1 Electrocompetent Cells
Phage Display Library Construction
MC1061 F-Electrocompetent Cells
Protein Expression
OverExpress Electrocompetent Cells
Phage Display Library Construction
SS320 (MC1061 F') Electrocompetent Cells
Competent cells for transformation
TransforMax EC100D Electrocompetent E. coli
Phage Display Library Construction
ER2738 Electrocompetent Cells
Large or Difficult Cloning
TransforMax EPI300-T1R Electrocompetent Cells
Phage Display Library Construction
TG1 Electrocompetent Cells
Phage Display Library Construction
MC1061 F-Electrocompetent Cells
Protein Expression
OverExpress Electrocompetent Cells
Phage Display Library Construction
SS320 (MC1061 F') Electrocompetent Cells
Competent cells for transformation
TransforMax EC100D Electrocompetent E. coli
Phage Display Library Construction
ER2738 Electrocompetent Cells
Product information
Introduction to ClearColi® technology:
Is there a better way to eliminate endotoxin contamination?
Now there is.
Instead of removing lipopolysaccharide (LPS) contamination from your protein or plasmid DNA preparations, eliminate the LPS at the source. Genetically modified LPS from a novel E. coli strain produces functionally clean recombinant proteins and plasmids. ClearColi® BL21(DE3) cells are the first commercially available competent cells with a modified LPS (Lipid IVA - see Fig. 1) that does not trigger the endotoxic response in mammalian cells. ClearColi™ cells lack outer membrane agonists for hTLR4/MD-2 activation; therefore, activation of hTLR4/MD-2 signalling by ClearColi is several orders of magnitude lower compared with E. coli wild-type cells, and heterologous proteins prepared from ClearColi are virtually free of endotoxic activity. After minimal purification from ClearColi cells, proteins or plasmids, which may still contain Lipid IVA, can still be used in most applications without eliciting an endotoxic response .
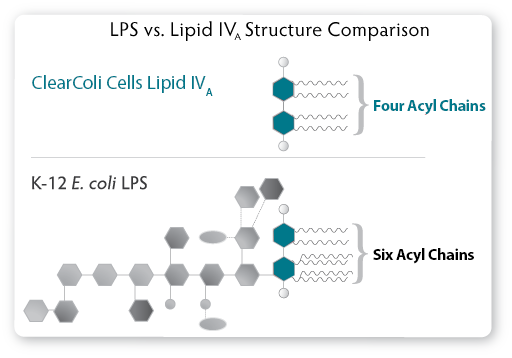 |
| Figure 1. The LPS of a normal E. coli cell compared to the genetically modified Lipid IVA from ClearColi cells. In ClearColi, the oligosaccharide chain has been deleted, and two of the six acyl chains have been removed to disable the endotoxin signal. |
Modifications to the genotype of the ClearColi cells consist of seven separate gene deletions, thereby ensuring there is no chance of genetic reversion back to wild type and production of normal LPS. These mutations result in the deletion of the oligosaccharide chain from the LPS, making it easier to remove the resulting lipid IVA from the downstream product. More importantly, two of the six acyl chains are deleted. The six acyl chains of the LPS are the trigger which is recognised by the Toll-like receptor 4 (TLR4) in complex with myeloid differentiation factor 2 (MD-2), causing activation of NF-ƙB and production of proinflammatory cytokines. Lipid IVA, which contains only four acyl chains, is not recognised by TLR4 and thus does not trigger the endotoxic response (see Figure. 2).
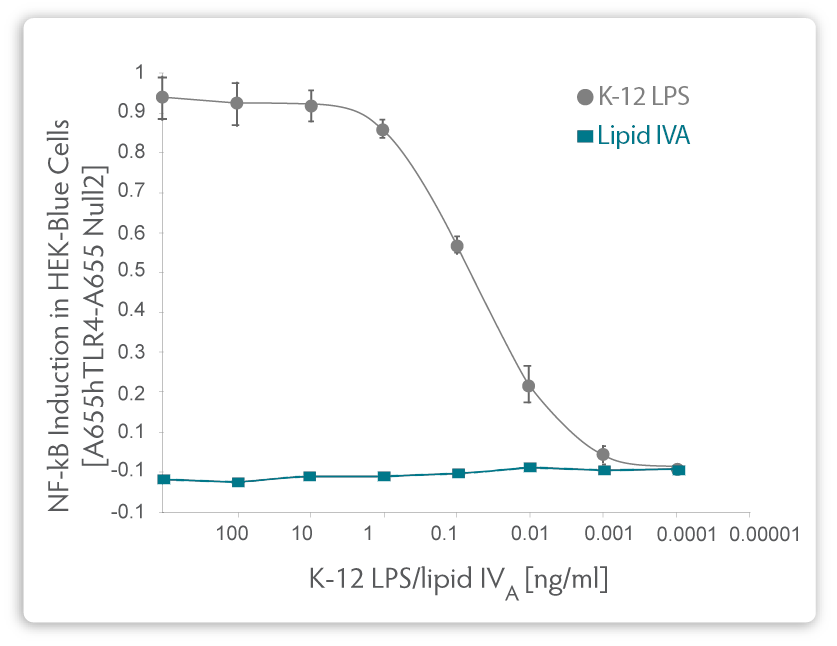 |
| Fig. 2. Comparison of relative NF-κB induction in HEK-Blue Cells using purified LPS from a K-12 E. coli strain or from pure, synthetically manufactured Lipid IVA. |
Genotype Information:
ClearColi BL21(DE3) competent cells have the following genotype:
F– ompT hsdSB (rB- mB-) gal dcm lon λ(DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) msbA148 ΔgutQ ΔkdsD ΔlpxLΔlpxMΔpagPΔlpxP ΔeptA
Seven specific deletion mutations (ΔgutQ ΔkdsD ΔlpxL ΔlpxMΔpagPΔlpxPΔeptA) encode the modification of LPS to Lipid IVA, while one additional compensating mutation (msbA148) enables the cells to maintain viability in the presence of the LPS precursor lipid IVA.
Why Endotoxin Removal is not the Best Method
Lipopolysaccharide (LPS), also known as endotoxin, is a potent agonist for hTLR4/MD-2-mediated proinflammatory activity in human immune cells. To prevent toxicity, recombinant proteins, commonly manufactured in E. coli, must be essentially free of endotoxin. However, efficient elimination of endotoxin is a challenging task, and none of the downstream applications remove endotoxin entirely. Current methods for endotoxin removal from recombinant proteins are varied, including ultra-filtration, activated carbon, surfactants, anion exchange chromatography, and immobilized sepharose. Each of these strategies involves negative effects: significant yield loss, high cost, loss of bioactivity of the protein, or bioactivity of the additives used for endotoxin clean up. Table. 1 below describes some of the most common methods and their associated shortcomings:
| Table 1: | |
| Method | Disadvantages |
| Ultrafiltration |
|
| Activated carbon |
|
| Surfactants |
|
| Anion-exchange chromatography |
|
| Histamine- and histidine-immobilized Sepharose |
|
| Polymyxin B-immobilised Sepharose |
|
Protein Expression with ClearColi
The relative production efficiency of endotoxin free ClearColi™ BL21(DE3) competent cells was compared to normal BL21(DE3) cells using two different recombinant proteins. Although overall growth rates of the ClearColi are slower, final protein production levels are very similar (see Figure. 3 and 4).
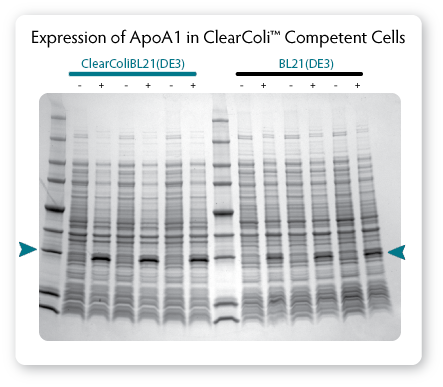 |
| Figure 3. Comparison of protein expression in ClearColi BL21(DE3) and Lucigen's E. Cloni® EXPRESS BL21(DE3) competent cells. Cells containing a T7 expression plasmid harbouring a gene encoding the human apolipoprotein A1 (ApoA1) were grown in LB Miller medium at 37 °C. When cultures reached OD600 of 0.6 to 0.8, expression was induced by the addition of 0.4 mM IPTG and incubation was continued for 3 hours. Equivalent numbers of uninduced (-) and induced (+) cells were lysed by heating in Laemmli buffer and samples were analysed by SDS-PAGE on a 4% - 20% polyacrylamide gradient gel. |
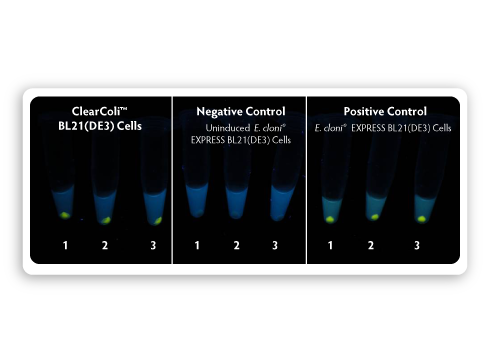 |
| Figure 4. Comparison of fluorescent protein expression in ClearColi BL21(DE3) and E. cloni EXPRESS BL21(DE3) cells. A pET expression vector harbouring a gene encoding a yellow fluorescent protein (LucY) under the control of a T7 promoter was transformed into both ClearColi BL21(DE3) and E. cloni EXPRESS BL21(DE3). Colonies were inoculated into LB-Miller medium and grown at 37 °C for induction. Samples of induced and uninduced cells containing equivalent numbers of cells were centrifuged, and cell pellets were photographed under long-wavelength UV illumination. |
Endotoxicity Assay Results
In most cases, a simple nickel column purification is sufficient for endotoxic shock response negative recombinant proteins suitable or downstream eukaryotic cellular response assays. Researchers can now assay target protein effects without concern that immune response triggers are a result of LPS endotoxin contamination. To demonstrate, ApoA1 protein expressed from ClearColi BL21(DE3) competent cells was Ni-column purified and tested for endotoxicity using both the HEK-Blue cell line from Invivogen (see Fig 5) and the LAL assay (see Fig. 6).
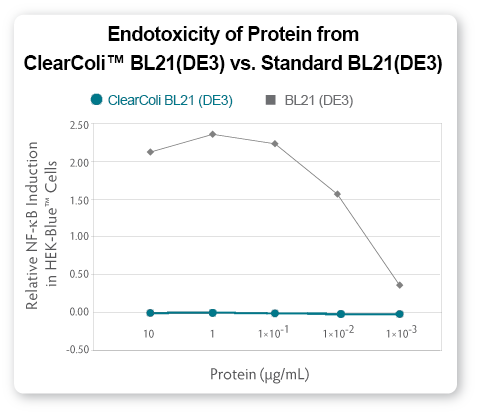 |
| Figure 5. Comparison of endotoxic response from protein derived from ClearColi BL21(DE3) and traditional BL21(DE3) competent cells |
LAL Testing: Does it really measure endotoxin?
Limulus amebocyte assay testing is an FDA-approved method for detection of endotoxins and the most common assay used; however the LAL assay is activated solely by the 4´-monophosphoryldiglucosamine backbone of LPS. LAL activity is minimally influenced by acylation pattern of LPS, the key determinant of endotoxicity in eukaryotic cells. The LAL assay also recognizes a wider spectrum of LPS/lipid A variants than the central cellular endotoxin sensor system of the human immune cell system. As such, false positive results can and will result due to the lack of specificity of the assay.
A simple Ni-column purification step for proteins produced from ClearColi cells will reduce LAL response levels by 95% or greater (see Fig. 6). However, the residual EU measurements are due to the non-specific nature of the assay unless extraneous LPS contamination from other sources is present. Alternative toxicity assays, such as those using HEK-Blue cells (see Fig. 5) suggest that even in the presence of EU levels above threshholds normally targeted by researchers, the actual immunogenic effects from ClearColi-derived proteins are non-existent.
Due to the non-specificity of the LAL assay when combined with lipid IVA from ClearColi, it is suggested that researchers consider alternative methods of endotoxin measurement.
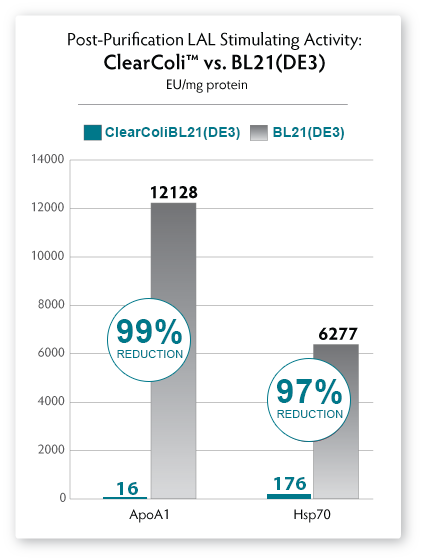 |
| Figure 6. Comparison of endotoxin units as measured by the LAL assay using nickel-column purified recombinant ApoA1 and HSP protein expressed in ClearColi BL21(DE3) (red bars) and E. cloni EXPRESS BL21(DE3) (grey bars) competent cells. Protein expressed from ClearColi demonstrates significant reduction in EU/mg without endotoxin removal steps. |
Growth Rates for ClearColi BL21(DE3) Cells
ClearColi BL21(DE3) cells grow at approximately 50% of the rate of normal BL21(DE3) cells (see Fig. 7). Users should expect to see very small colonies for the first 24 hours after plating transformants. We recommend incubating plates for 32-40 hours before picking colonies for future experiments. When grown to sufficient densities, ClearColi BL21(DE3) cells produce similar protein levels as normal BL21(DE3) cells when comparing equal numbers of cells.
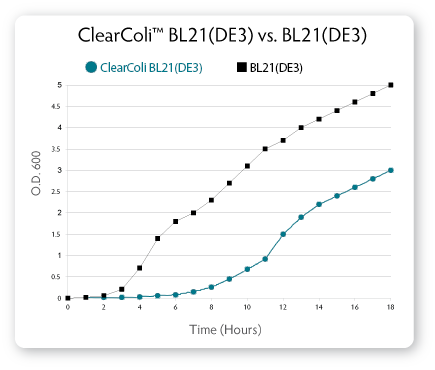 |
| Figure 7. Comparison of growth rates for ClearColi BL21(DE3) vs. E. cloni EXPRESS BL21(DE3) cells. Cells were inoculated to an initial OD600 of ~0.003 in 200 ml of LB Miller medium and grown at 37° C with shaking at 210 rpm. The OD600 of the cultures was recorded hourly. |
Relevant Reference Articles:
- Teghanemt, et al, Molecular Basis of Reduced Potency of Underacylated Endotoxins, J Immunol, 2005; 175:4669-4676
- Mamat, et al, Single amino acid substitutions in either YhjD or MsbA confer viability to 3-deoxy-D-manno-oct-2-ulosonic acid-depleted Escherichia coli, Molecular Microbiology, 2008, 67(3), 633–648
- Meredith, et al, Redefining the Requisite Lipopolysaccharide Structure in Escherichia coli, ACS Chemical Biology, 2006, 1(1), 33-42
- Brandenburg, et al, The Expression of Endotoxic Activity in the Limulus Test as Compared to Cytokine Production in Immune Cells, Current Medicinal Chemistry, 2009, 16, 2653-2660
- Gutsmann, et al. Structural prerequisites for endotoxic activity in the Limulus test as compared to cytokine production in mononuclear cells, Innate Immunity, 2010, 16(1), 39-47
- Beom Seok Park1 et al., The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex, Nature 458, 1191-1195 (30 April 2009)
ClearColi Licensing Information:
ClearColi™ Competent cells are subject to US Patent 8,303,964 and other US and foreign pending patents.
Lucigen Corporation ("Lucigen") has a license from Research Corporation Technologies to sell ClearColi competent cells to third-parties for non-commercial research purposes only. A separate license is required for any commercial use. For more information about the use of this product by commercial entities, please review our full licensing page.
Related Products
Size
E. cloni 10G Chemically Competent Cells
Size
E. cloni EXPRESS Competent Cells
General Cloning and Library Construction
HI-Control 10G Chemically Competent Cells
Protein Expression
HI-Control BL21(DE3) Chemically Competent Cells
Protein Expression
OverExpress Chemically Competent Cells
General Cloning and Library Construction
E. cloni 5-alpha Chemically Competent Cells
Large or Difficult Cloning
CopyCutter EPI400 Competent Cells
Format
Endura Competent Cells
Format
TransforMax EC100 Competent Cells
Size
E. cloni 10G Chemically Competent Cells
Size
E. cloni EXPRESS Competent Cells
General Cloning and Library Construction
HI-Control 10G Chemically Competent Cells
Protein Expression
HI-Control BL21(DE3) Chemically Competent Cells
Protein Expression
OverExpress Chemically Competent Cells
General Cloning and Library Construction
E. cloni 5-alpha Chemically Competent Cells
Large or Difficult Cloning
CopyCutter EPI400 Competent Cells
Format
Endura Competent Cells
Format
TransforMax EC100 Competent Cells
Size
E. cloni 10G Chemically Competent Cells
Size
E. cloni EXPRESS Competent Cells
Transformation efficiency:
1 × 109 cfu/ug, measured using pUC19 DNA in standard electroporation conditions
F– ompT hsdSB (rB- mB-) gal dcm lon λ(DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) msbA148 ΔgutQΔkdsD ΔlpxLΔlpxMΔpagPΔlpxPΔeptA
SDS
Manuals and user guides
Product information sheets
FAQs
You may also be interested in...
Large or Difficult Cloning
CopyCutter Induction Solution
Size
TransforMax EPI300
Large or Difficult Cloning
CopyControl Induction Solution
Recovery Media
Expression Recovery Medium
Large or Difficult Cloning
CopyCutter Induction Solution
Size
TransforMax EPI300
Large or Difficult Cloning
CopyControl Induction Solution
Recovery Media
Expression Recovery Medium
Large or Difficult Cloning
CopyCutter Induction Solution
Size
TransforMax EPI300
Access support
Need some support with placing an order, setting up an account, or finding the right protocol?
Contact us