In sequencing applications, the design of primers can be complicated by the degeneracy of the genetic code (there are 64 possible 3-base codon configurations and only 21 amino acids, and therefore the third base in a sequence codon is often unknown). The problem of degeneracy can also be tackled by the use of universal bases.(1) Deoxyinosine is often used as a degenerate base in an oligonucleotide to alleviate this problem.(2) This is possible since its structure allows it to base pair with all four bases in various ‘wobble’ structures. However, the base-pairing is not equivalent with each of the 4 naturally occurring bases. The overall preferential order of base-pairing is: dI-dC > dI-dA > dI-dG = dI-dT. We provide both dI phosphoramidite and CPGs. 2'-Deoxynebularine(3) is another example.
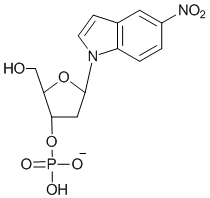
In a true universal nucleoside, the base analogue does not hybridize significantly to the other four bases and makes up some of the duplex destabilization by acting as an intercalating agent. 3-nitropyrrole 2'-deoxynucleosides were the first example of a set of universal bases. Subsequently, 5-nitroindole was determined to be an effective universal base and to be superior to 3-nitropyrrole, based on duplex melting experiments.
Ref:
- The applications of universal DNA base analogues, D. Loakes, Nucleic Acids Research, 29, 2437-2447, 2001.
- (a) Base pairing involving deoxyinosine: implications for probe design, F.H. Martin, M.M. Castro, F. Aboul- ela and I. Tinoco, Jr, Nucleic Acids Research, 13, 8927-8938, 1985; (b) Studies on the base pairing properties of deoxyinosine by solid phase hybridisation to oligonucleotides, S.C. Case-Green, E.M. Southern, Nucleic Acids Research, 22, 131-136, 1994.
- (a) Synthesis and properties of oligonucleotides containing 2'-deoxynebularine and 2'-deoxyxanthosine, R. Eritja, D.M. Horowitz, P.A. Walker, J.P. Ziehler-Martin, M.S. Boosalis, M.F. Goodman, K. Itakura and B.E. Kaplan, Nucleic Acids Research, 14, 8135-8153, 1986; (b) As a custom item we have prepared the phosphoramidite, see: A convenient synthesis of deoxynebularine phosphoramidite, D. Picken and V. Gault, Nucleosides, Nucleotides and Nucleic Acids, 16, 937-939, 1997. Please enquire regarding availability.