5'-DMT-mdC(TEG-NH-Fmoc)-Suc-CPG Column
5'-DMT-mdC(TEG-NH-Fmoc)-Suc-CPG Column
Key features
Show- Incorporates a primary amine at the 3 end of an oligonucleotide, for subsequent conjugation.
- Does not block the terminus from any desired enzymatic activity.
- Base-labile trifluoroacetyl (TFA) protection on the amine, which is removed during cleavage and deprotection.
- Triethylene glycol spacer confers additional hydrophilicity.
- CPG has aminopropyl-succinyl linker.
Product information
Incorporation of a primary amine reactive functional group at specific sites within an oligonucleotide allows for subsequent post-synthesis conjugation of the oligo with a number of different affinity, reporter or protein labels, depending on the application. Such labels need to be reactive towards the incorporated functional group: for example, NHS esters or isothiocyanates will react with primary amines. This approach is often necessary where the desired label or tag is either not available as a phosphoramidite, or is sensitive or unstable to the conditions of oligonucleotide synthesis or deprotection. A common example is the attachment of a rhodamine dye using the TAMRA NHS ester. Functionally-derivatised oligos can also be covalently attached to surfaces such as glass slides or gold microspheres for use in various microarray or nanoelectronic applications. The most commonly used product for introducing a 3'-amino functionality is Fmoc-protected 3'-amino C7 CPG. Use of an Fmoc-protected amine has both advantages and disadvantages. This is quite stable to oligo synthesis conditions however, if not handled correctly, some loss of Fmoc may occur. This leads to capping of the free amine with acetic anhydride and hence loss of functionality.
The main advantage of Fmoc is that it can be removed selectively without cleavage from the support allowing solid-phase conjugation of the desired label. This can be done prior to, or subsequent to, oligonucleotide synthesis. Alternatives to Fmoc protection have been investigated. Phthalimide (PT) chemistry has been used in the development of 3'-PT-amino-C6 CPG (1), where the nitrogen that will ultimately provide the 3'-amino function is part of the PT group attached to the support through an imide group attached to the aromatic ring. This linkage is stable to all conditions of oligo synthesis and the resulting amino functionality does not add any additional chiral centres/diastereomers to the oligo. A completely analogous C3 product is also available. Additional products are available for introducing 3'-amino functionality without blocking the terminus from any desired enzymatic activity. There are the C6 dC and dT analogues, plus the mdC TEG product confers additional spacing and hydrophilicity. These products all have base-labile trifluoroacetyl (TFA) protection on the amine, which is removed during cleavage and deprotection.
Ref:
- An improved CPG support for the synthesis of 3'-amine-tailed oligonucleotides, C.R. Petrie, M.W. Reed, A.D. Adams and R.B. Meyer, Jr., Bioconjugate Chem., 3, 85-87, 1992.
|
Synthesizer
|
Column
|
Type/Description
|
Notes
|
|---|---|---|---|
|
MerMade 6,12
|
MerMade, syringe (all scales)
|
Pipette type column
|
A MerMade column is also known as a Supercolumn
|
|
MerMade 4, 48X, 96E, 192E, 192X
|
MerMade, Syringe (up to 1.3 mL)
|
Pipette type column
|
A MerMade column is also known as a Supercolumn
|
|
ABI 384 / 394
|
Luer
|
Barrel column with luer fitting at either end
|
Also known as ALL-FIT or Standard
|
|
Expedite 8909
|
Luer
|
Barrel column with luer fitting at either end
|
Also known as ALL-FIT or Standard
|
|
ABI3900
|
MerMade
|
Pipette type column
|
A MerMade column is also known as a Supercolumn
|
|
K&A H4, H8, H8SE, H2, H32, H64
|
Luer
|
Barrel column with luer fitting at either end
|
Also known as ALL-FIT or Standard
|
|
K&A S4CL/S8CL
|
Luer
|
Barrel column with luer fitting at either end
|
Also known as Standard. For this instrument, we recommend the Luer (Standard) column as the ALL-FIT columns have a wider barrel.
|
|
Dr Oligo 48
|
MerMade
|
Pipette type column
|
A MerMade column is also known as a Supercolumn
|
|
Dr Oligo 192XLc, 768XLc just plates
|
MerMade, Syringe (up to 1.3 mL)
|
Pipette type column
|
A MerMade column is also known as a Supercolumn
|
|
OligoMaker X12, 48, 192, X192, X96
|
MerMade, Syringe (up to 1.3 mL)
|
Pipette type column
|
A MerMade column is also known as a Supercolumn
|
Properties:
- Appearance: White Powder
Product usage:
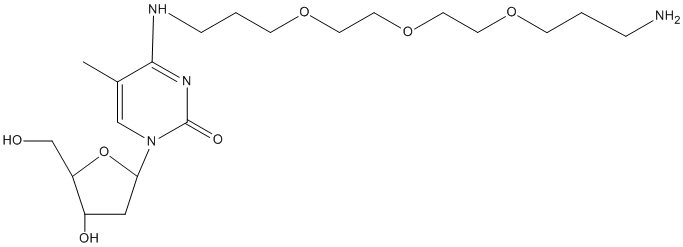
- Deprotection conditions: To avoid a secondary alkylation acryonitrile reaction, which occurs during normal cleavage and deprotection of amine modified oligos, flush column for 15 minutes with 1 mL of 10% Diethylamine (Aldrich #471216) in MeCN and rinse column with MeCN before cleavage and deprotection of the amine labeled oligo.Cleave and deprotect with methylamine/ammonia (1:1) for 2 hours at 60 °Cwhen using fast deprotecting amidites (i.e. C-Ac, G-DMF). With standard amidites (i.e. C-Bz, G-iBu) use concentrated ammonia for 5 hours at 60 °C. Amine Labeling:If labeling the 3' terminus, the DMT group may be left the 5'terminus on after the synthesis for reverse-phase cartridge purification.Oligo should be cleaved, deprotected, and purified before labeling. For reporter groups, protein labels, NHS, and enzymes see manufacturer's labeling protocol.Image of cleaved and deprotected structure:
- The mass this product adds after conjugation and work-up (the additional mass seen by mass spectrometry) is: 506.48
Storage and handling:
- Shipping conditions: Ambient
- Storage conditions: -15 to -30 °C in sealed container
Access support
Need some support with placing an order, setting up an account, or finding the right protocol?
Contact us