The DuraScribe® T7 Transcription Kit* is an in vitro transcription kit that produces RNA - called DuraScript® RNA - that is completely resistant to RNase A digestion.
The DuraScribe T7 Transcription Kit includes DuraScribe T7 RNA Polymerase, an enhanced formulation of the Y639F mutant 1 of T7 RNA Polymerase that efficiently incorporates 2´-Fluorine-CTP (2´-F-dCTP), 2´-Fluorine-UTP (2´-F-dUTP), ATP and GTP into RNase-resistant RNA transcripts called DuraScript RNA (Figure 1). The DuraScribe T7 RNA Polymerase uses the same T7 promoters as the wild-type T7 RNA Polymerase.
Applications
DuraScript RNA has demonstrated advantages for RNA aptamer synthesis, and ribozyme, RNA interference (RNAi) and antisense RNA studies.
Features
-
DuraScript RNA is resistant to RNase A and DNase
-
High Yields of DuraScript RNA
-
T7 in vitro transcription reactions. DuraScribe T7 RNA Polymerase uses the same T7 promoters as the wild type T7 RNA Polymerase.
-
Ideal for RNA aptamer studies. Numerous citations using the DuraScribe Kit with RNA aptamers
-
SELEX-compatible. DuraScript RNA can be used in SELEX selection processes.
-
Make long or short DuraScript RNA. Double-strand oligos, linearized plasmids, and PCR products with a T7 polymerase promoter can be transcribed.
Table 1. Yield of DuraScript RNA from a DuraScribe Kit reaction. One microgram of a 3-Kb DNA template was linearised at different sites and then transcribed in a DuraScribe T7 Transcription Kit reaction for 4 hours. The yield of DuraScript RNA produced from each template is shown in micrograms (µg) and in picomoles (pmol).
|
Size of DuraScript RNA produced
|
DuraScript RNA Yield (µg)
|
DuraScript RNA Yield (pmol)
|
| 2600 nts |
100 µg |
116 pmol |
| 1400 nts |
58 µg |
124 pmol |
| 330 nts |
18 µg |
164 pmol |
| 88 nts |
9 µg |
307 pmol |
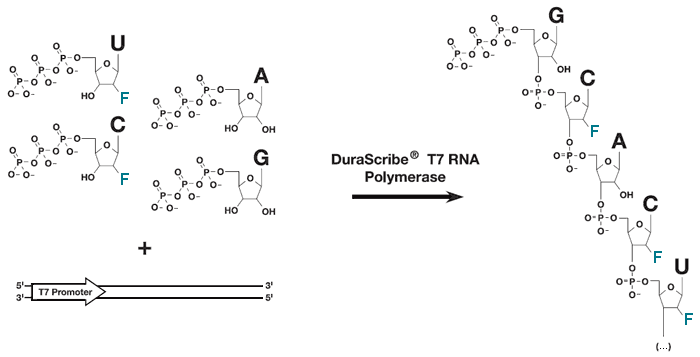
Figure 1 (click to enlarge). The DuraScribe T7 RNA Polymerase efficiently incorporates 2´-F-dCTP and 2´-F-dUTP into full-length DuraScript RNA. The presence of the fluorine at the 2´-position of the 2´-F-dC and 2´-F-dU nucleotides prevents digestion by RNase A.
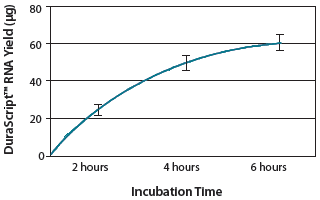
Figure 2. Yield of RNA from a DuraScribe® T7 Transcription reaction. A standard reaction (4-6 hours) produced 40-60 µg of a 1.4-kb DuraScript® RNA.
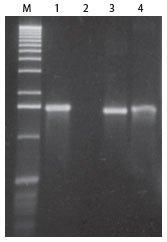
Figure 3. DuraScript® RNA is resistant to RNase A digestion. A 1.4-kb standard RNA transcript and a 1.4-kb DuraScript RNA transcript were each incubated with 1 U of highly purified RNase A for 30 minutes. The standard RNA transcript was completely degraded while the DuraScript RNA transcript remained intact. Lane M, size ladder; lane 1, 1.4-kb standard RNA transcript; lane 2, standard RNA after RNase A treatment; lane 3, 1.4-kb DuraScript RNA; lane 4, DuraScript RNA after RNase A treatment. Denaturing 1% agarose gel 2.

Figure 4. DuraScript RNA is completely resistant to "finger" nucleases. A 1.4-kb standard RNA transcript and a 1.4-kb DuraScript RNA transcript were produced using sterile water or water that had been contaminated by exposure to the hands of a test subject. The standard RNA shows extensive degradation from "finger" nucleases in the contaminated water while the DuraScript RNA remains fully intact. M, Size ladder; Lane 1, Standard RNA transcript; Lane 2, Standard RNA after "finger" nuclease exposure; Lane 3, DuraScript RNA; Lane 4, DuraScript RNA after "finger" nuclease exposure. Denaturing 1% agarose gel 2.
* Covered by issued and/or pending patents.
References
- Sousa, R. and Padilla, R. (1995) EMBOJ. 14:18 4609-4621.
- Molecular Cloning - A Laboratory Manual, Third Edition, 2001. CSHL Press. pp 7.27 - 7.34. J. Sambrook and D. Russell.



