T4 Polynucleotide Kinase, Cloned
Product Details
ShowEach T4 Polynucleotide Kinase Kit includes T4 Polynucleotide Kinase at 10 U/µL and 10X Reaction Buffer without ATP. ATP is available separately.
The "enzyme only" format contains T4 Polynucleotide Kinase at 10 U/µL.
T4 Polynucleotide Kinase, Cloned
Key features
Show- Phosphorylate 5' hydroxy ends of ssDNA and dsDNA, RNA, and nucleoside 3 monophosphates
- Highly active enzyme that readily phosphorylates 5' hydroxyl ends of nucleic acids
- Flexible: Use newly phosphorylated nucleic acids in a variety of applications such as cloning, next gen sequencing library prep, and preparation of labelled nucleic acids
Product information
T4 Polynucleotide Kinase (PNK) catalyses the transfer of the γ-phosphate from ATP to the 5´ hydroxyl of ssDNA and dsDNA, RNA, and nucleoside 3´ monophosphates. The enzyme also removes the 3´ phosphate from 3´-phosphoryl polynucleotides, deoxyribonucleoside 3´ monophosphates, and deoxyribonucleoside-3´,5´-diphosphates to form a 3´-hydroxyl group.
Applications
- Labeling of 5´ termini of DNA and RNA with 32P or 33P for DNA sequencing, blot-hybridization experiments, or transcript mapping using Mung Bean Nuclease, S1 nuclease, or other nucleases.1,2
- Phosphorylation of oligonucleotide linkers and other DNA or RNA molecules prior to ligation, or for use in ligation amplification reactions with Ampligase® Thermostable DNA Ligase.
- Preparation of labelled DNA or RNA molecular weight markers for gel electrophoresis and chromatography.
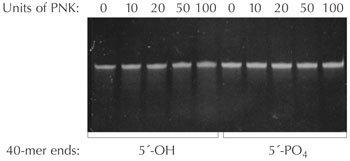
Figure 1. The absence of DNA exonuclease activity in Epicentre's T4 Polynucleotide Kinase. 40-mer oligonucleotides with (5´-PO4) or without (5´-OH) phosphorylated 5´-termini were incubated with the indicated number of units of T4 PNK in 1X T4 PNK Reaction Buffer for 16 hours at 37°C. Electrophoresis of the incubation mixtures in a 15% polyacrylamide/8 M urea gel demonstrates no degradation of the oligonucleotides.
References
- Sambrook, J. et al. (1989) in: Molecular Cloning: A Laboratory Manual (2nd ed.), Cold Spring Harbor Laboratory Press, New York.
- Chaconas, G. et al. (1980) Meth. Enzymol. 65, 75.
Access support
Need some support with placing an order, setting up an account, or finding the right protocol?
Contact us